ReCET™
Resetting the metabolic
operating system
Signaling a new era in type 2 diabetes therapy
Research shows that signaling from the small intestine (specifically the duodenum) is a key regulator of metabolic function, including blood sugar management in the body.5,6 When signaling in the duodenum becomes abnormal, the body cannot properly regulate blood sugar, contributing to the development and progression of type 2 diabetes (T2D).
The ReCET System’s goal of resetting the metabolic operating system is a foundational therapy aiming to restore proper communication between the duodenum and the organs involved in metabolic function, which are key to changing the way type 2 diabetes is treated.
The ReCET Procedure is investigational and is currently being evaluated in clinical studies.
What is the ReCET System?
The ReCET System applies the principle of resetting the metabolic operating system by eliminating poorly functioning signaling cells inducing regrowth of healthy signaling cells in the duodenum with the intent to slow the progression of T2D, improve blood sugar control, and reduce dependence on medication.
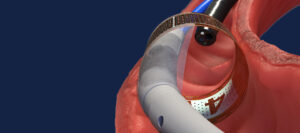
The ReCET Technology consists of a specialized catheter that delivers pulsed electric fields to the mucosa and sub-mucosa duodenal tissue, initiating the body’s natural process of cellular regeneration. This process is designed to promote stem cell directed regrowth of healthy cells to improve cellular signaling and the body’s ability to manage blood sugar levels.
The ReCET Procedure
The ReCET Procedure is investigational and is currently being evaluated in clinical studies.
While the patient is asleep, a physician uses a small flexible camera (an endoscope) to introduce the ReCET Catheter through the mouth and past the stomach to the first part of the small intestine (duodenum). A small coil is deployed from the ReCET Catheter and a controlled, electric current is applied to the mucosa and sub-mucosa lining of the duodenum.
This delivery of energy is designed to trigger the body’s natural process to regenerate healthy cells that may help control blood sugar levels and slow the progression of type 2 diabetes.

The ReCET Study is a pivotal clinical study evaluating the use of the ReCET System in people living with type 2 diabetes. The ReCET Study will be enrolling participants at clinical sites in the United States and Australia.
